KalVista’s KOMPLETE View
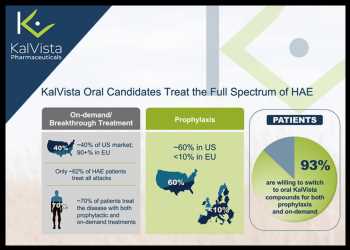
Hereditary angioedema or HAE in short, is a rare genetic disorder in which patients experience sudden onset of swelling in various locations of the body. There are two kinds of HAE treatments – preventive and on-demand. While preventive or prophylactic HAE treatment helps prevent or minimize the frequency and severity of HAE attacks, the on-demand HAE treatment is used to treat the symptoms of a HAE attack.
The company we are profiling today is KalVista Pharmaceuticals Inc. (KALV), which is developing a franchise of oral treatments for HAE – both on-demand and prophylactic HAE drugs.
KalVista’s on-demand HAE drug candidate KVD900 has been successfully tested in a phase II trial, with the drug candidate demonstrating a rapid onset of symptom relief and significant reduction in the use of rescue medication.
An end-of-phase II meeting with the FDA to review the proposed phase III program for KVD900 is expected to take place this month (September 2021).
The company’s prophylactic treatment of HAE under development is KVD824.
KVD824 has advanced to phase II trial in HAE, dubbed KOMPLETE, and patient enrollment in the trial is underway in Canada, Australia, and the UK. In April of this year, the FDA had placed a hold on the KOMPLETE trial, requesting further analysis related to preclinical studies to be made prior to the trial’s launch.
Now, after five months, the company received FDA permission yesterday (Sep.14) to continue dosing patients in the KOMPLETE trial. With the lifting of the FDA clinical hold, the execution of KOMPLETE is expected to accelerate as it can now proceed at its U.S. trial sites.
KOMPLETE will be conducted at more than 30 sites in 13 countries.
In December 2020, BioCryst Pharmaceuticals Inc.’s (BCRX) Orladeyo received FDA approval, becoming the first oral therapy to prevent HAE attacks.
KalVista’s direct competitor Pharvaris (PHVS) is also developing a drug for on-demand treatment and prophylactic prevention of HAE attacks. The drug candidate PHVS416 is under phase II trial for the on-demand treatment of HAE attacks and a phase II prophylactic study in HAE is expected to begin this year.
Also in the pipeline are KVD001 and Factor XIIa program.
— KVD001, being developed for Diabetic Macular Edema, has completed phase II testing. The next steps of development are being evaluated.
— Factor XIIa program as next generation of oral therapy for Hereditary Angioedema, is under preclinical development. This compound is advancing towards IND-enabling studies.
Cash position:
As of July 31, 2021, the company had cash of $230.6 million.
KALV has traded in a range of $12.01 to $45.00 in the last 1 year. The stock closed Tuesday’s trading at $19.11, down 5.21%.
Source: Read Full Article